08 / 26 / 2023
09:30 ~ 17:30
Xixi National Wetland Park
Invited scholars
West Lake Forum: The mechanism, evolution and influence of mutation
Back to ListOn August 26, 2023, the Center for Evolutionary & Organismal Biology at Zhejiang University organized an interdisciplinary forum focusing on evolutionary biology, molecular biology, and cell biology.
The symposium focused on the theme "Mechanisms, Evolutionary Patterns, and Impacts of Mutations." It was chaired by Professor Guojie Zhang from the Center for Evolutionary & Organismal Biology at Zhejiang University. Attendees included researchers such as Dr. Dongyi Xu from Peking University, Dr. Ying Zhang from Beijing Normal University, Drs. Jing Qu and Yong Zhang from the Institute of Zoology, Chinese Academy of Sciences, Dr. Jinchuan Hu from Fudan University, Dr. Yanmei Dou from Westlake University, Professors Jun Huang and Dong Fang from the Life Sciences Institute, Zhejiang University
, along with Dr. Shaohong Feng and Dr. Haoxuan Liu from the Center for Evolutionary & Organismal Biology at Zhejiang University.
DNA damage repair
As the genetic material of life, DNA replication is an exceptionally precise biological process. The entire replication process is tightly regulated to ensure the accurate replication of each DNA base, preventing erroneous insertions or deletions. Additionally, DNA is constantly subjected to damage from both internal and external environmental factors. In order to protect the integrity of the genome, life has evolved an intricate and sophisticated set of mechanisms. Professor Jun Huang from the Life Sciences Institute, Zhejiang University, provided a detailed explanation of the DNA damage repair pathways in humans.
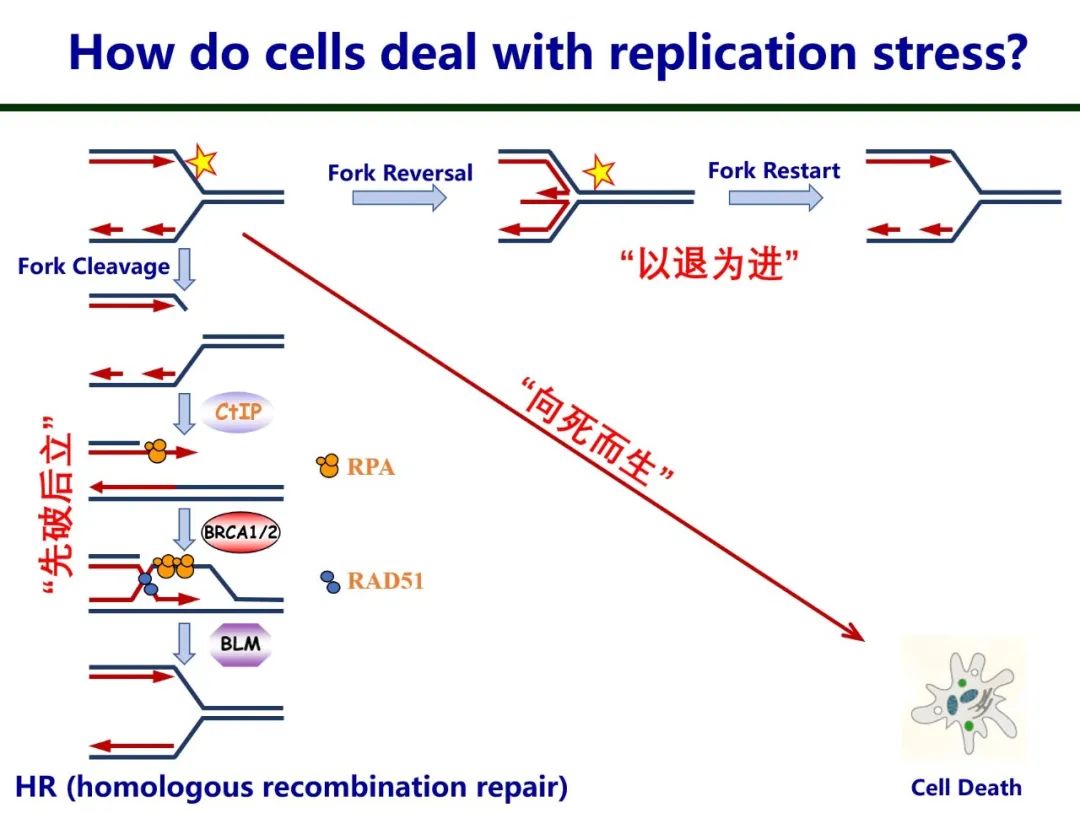
In a human cell, an average of approximately seventy thousand different types of damage occur per cell per day. Faced with this replication stress, cells employ three coping strategies. First is "break and repair," where the cell cuts the damaged site and uses homologous recombination to repair it. Second is the replication fork reverses at the damaged site to stabilize stalled replication forks. Lastly, "death to survive" occurs when damaged sites cannot be repaired, triggering the cell's apoptosis mechanism to eliminate damaged cells. Malfunctions in the body's repair mechanisms often lead to severe conditions such as Fanconi Anemia, Seckel Syndrome, and Bloom's Syndrome.

Professor Jun Huang from Life Sciences Institute, Zhejiang University
Research into DNA damage repair pathways offers potential for treating various diseases, including cancer. Dr. Dongyi Xu from Peking University's School of Life Sciences presented the classic approach using PARP inhibitors to treat cancer. Mutations in the BRCA gene are prevalent driver mutations in cancer, commonly found in breast and ovarian cancers. The BRCA gene is integral to the homologous recombination repair pathway. When this gene is mutated and inactivated, cells accumulate numerous mutations, promoting carcinogenesis. Inhibiting the parallel pathway in these cancer cells with PARP inhibitors prevents DNA damage repair, leading to cellular apoptosis. Consequently, PARP inhibitors are extensively used as anticancer drugs in clinical settings, showing promising results. However, these drugs are not flawless. Similar to many targeted therapies, prolonged use can prompt cancer cells to develop drug resistance. The mechanism involves the emergence of new mutations in cancer cells, reactivating the homologous recombination pathway to repair damage.
Dr. Dongyi Xu from the School of Life Sciences, Peking University
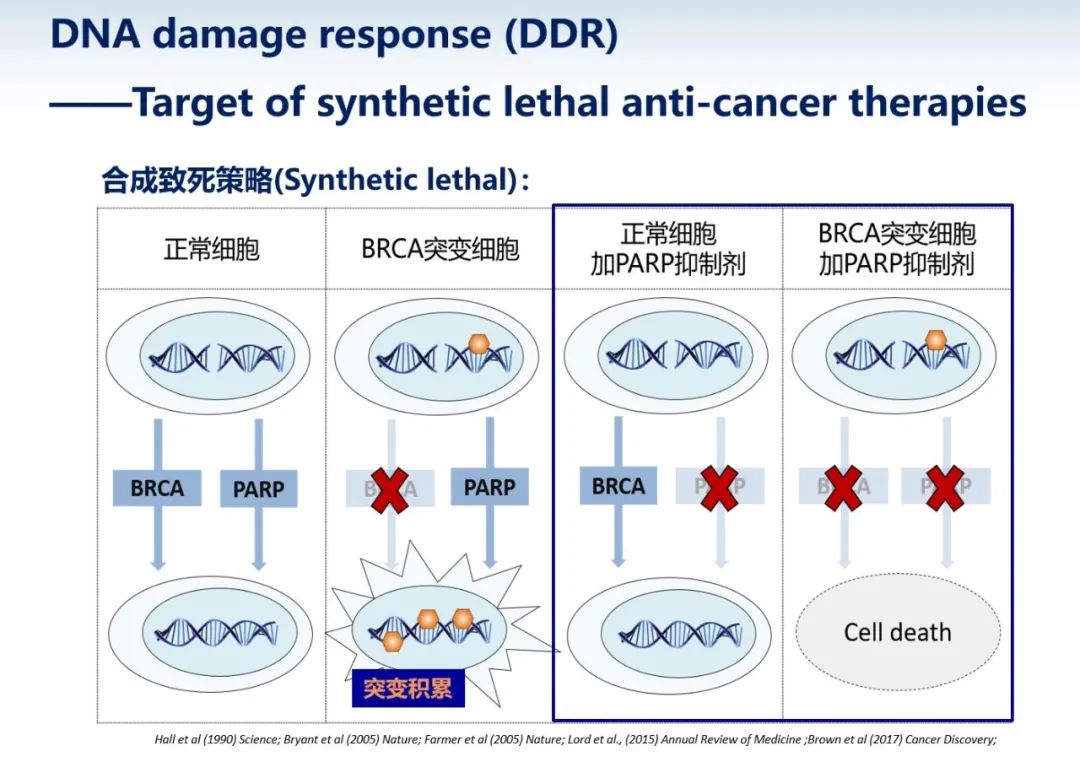
The anticancer mechanism of PARP inhibitors
The prerequisite for studying DNA damage repair is to understand where these repair events occur in the genome. Dr. Jinchuan Hu from the Biomedical Research Institute at Fudan University introduced a method developed by his team that accurately locates damage repair events. Damage-seq and XR-seq can pinpoint DNA adducts and nucleotide excision repair, providing a mapped distribution of the genome. Widely cited in the scientific community, these methods have driven advancements in the field of DNA damage repair research. Building upon this foundation, Dr. Hu's team pioneered the use of ChIP in combination with Damage-seq, achieving the first-ever detection of protein-DNA damage interactions at single-nucleotide resolution, thereby advancing research into the mechanisms of this area.

Dr. Jinchuan Hu from the Biomedical Research Institute at Fudan University
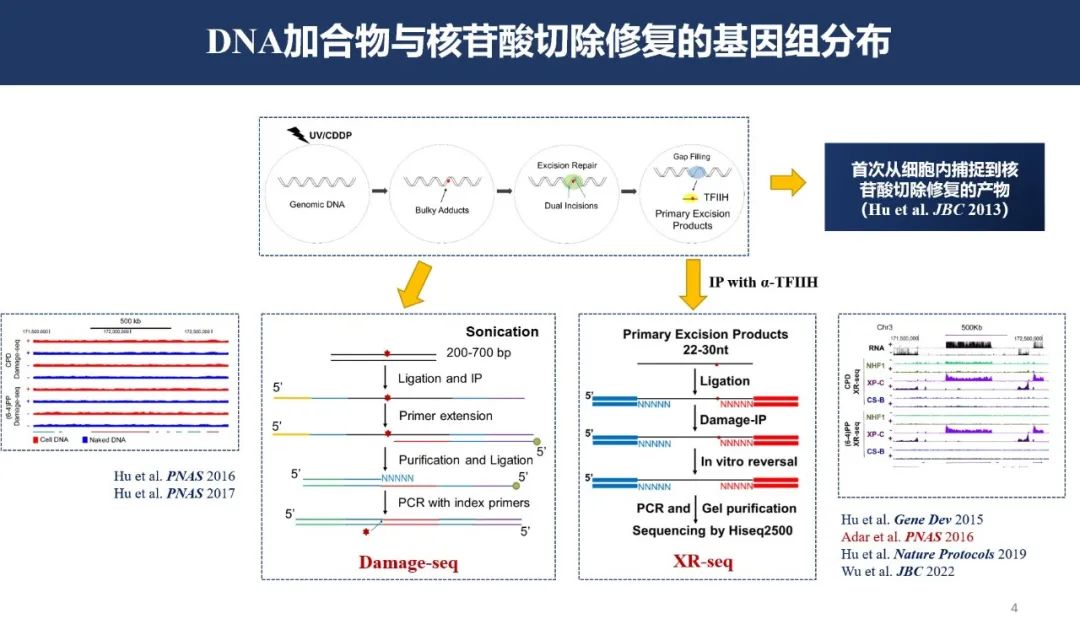 Damage-seq and XR-seq
Damage-seq and XR-seq
The mutations in germ cells and somatic cells
Mutations retained during DNA replication or repair are known as mutations. Mutations are categorized based on their heritability into germ cell mutations and somatic cell mutations. In higher animals, there's differentiation between the reproductive system and other systems. Mutations occurring in the reproductive system, capable of being passed on to offspring, are termed germ cell mutations. Those occurring in other bodily systems that cannot be inherited by offspring are called somatic cell mutations. Germ cell mutations play a crucial role in species evolution, and their frequency is widely studied. The mutation rate in germ cells is often measured in mutations per site per generation or mutations per site per year.
Professor Guojie Zhang from the Center for Evolutionary& Organismal Biology, Zhejiang University, presented his team's work in this field. First, he clarified that the mutation rate, as an important characteristic, is regulated by other life traits and evolves continuously. Previous studies on the reproductive cell mutation rates of vertebrates were mostly confined to individual species due to the extensive workload involved in experiments and analysis. As of 2022, the known vertebrates with documented reproductive cell mutation rates numbered around 20 species. Professor Zhang's team conducted extensive experiments, measuring the reproductive cell mutation rates of 68 vertebrate species at once, significantly amplifying the data available in this field. Based on this, they could analyze the influencing factors and evolutionary patterns of reproductive cell mutation rates. This research has unveiled substantial differences in mutation rates among vertebrates, exceeding 125-fold differences between different species (per site/year). More importantly, the study identified certain parameters closely associated with reproductive cell mutation rates that correlate with life history traits, such as generation time, sexual maturity time, litter size, and effective population size.

Professor Guojie Zhang from the Center for Evolutionary& Organismal Biology, Zhejiang University
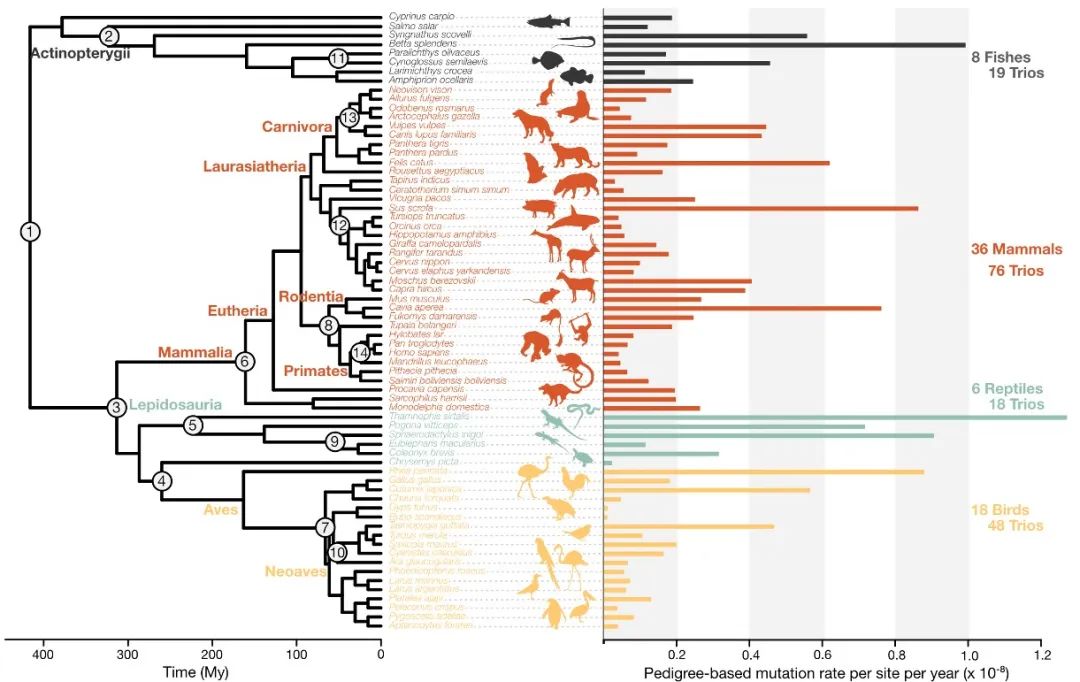
The differences in reproductive cell mutation rates among vertebrates
Somatic mutations, though not heritable to offspring, have significant implications for an individual's overall health. For instance, over 7000 diseases are attributed to somatic mutations, and they are closely associated with aging. Dr. Yanmei Dou from Westlake University presented her team's work on identifying and applying somatic mutations. While germline mutations pass to every cell in offspring, somatic mutations exist in only a subset of cells in an individual, leading to varying proportions or tissues carrying these mutations, depending on when they occur during development. This characteristic complicates the identification of somatic mutations. Dr. Yanmei Dou introduced their developed algorithm called MosaicForecast, utilizing machine learning to identify somatic mutations accurately within Next-Generation Sequencing (NGS) data, far surpassing other algorithms in accuracy. By identifying somatic mutations, researchers can trace their developmental relationships and estimate that each cell division in the earliest stages of development may lead to 2-3 somatic mutations. These studies play a crucial role in explaining the relationship between somatic mutations and human health.
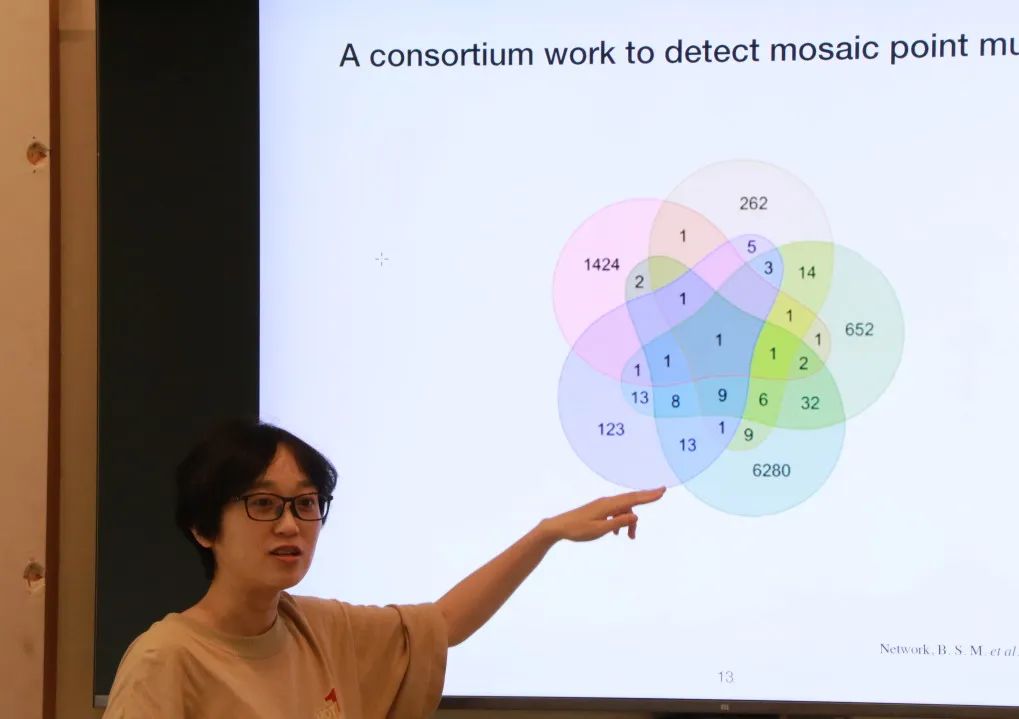
Dr. Yanmei Dou from Westlake University
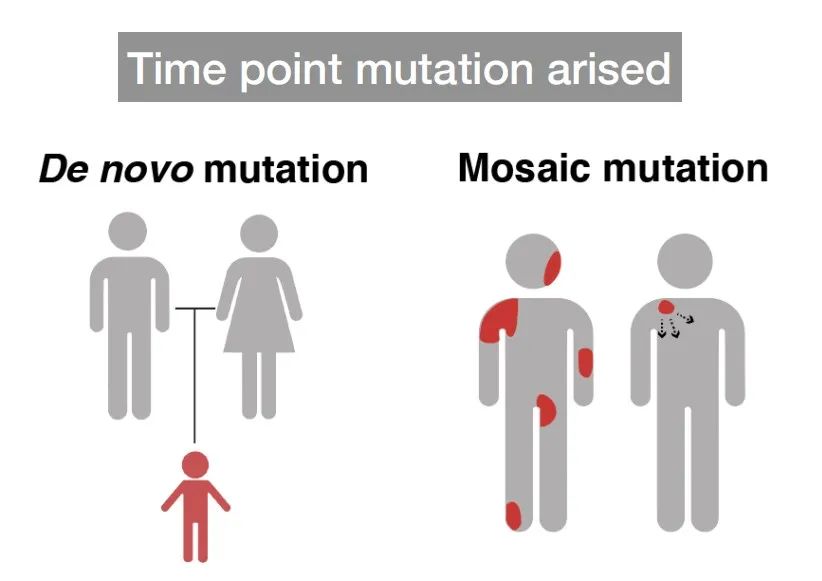
The mutations in germ cells and somatic cells
The evolutionary driving forces behind mutation rates
In the preceding context, the mutation rate, as a significant trait, follows its own evolutionary patterns throughout the continuous process of evolution. Typically, the level of a trait is determined by the evolutionary driving forces acting upon it. So, what are the evolutionary driving forces that impact the mutation rate? Dr. Haoxuan Liu from the Center for Evolutionary & Organismal Biology shared insights on this topic. Because most mutations are harmful, these detrimental effects drive the mutation rate downward, which is why the mutation rates across all species are currently at very low levels (<10-7 /site/generation). Apart from harmful mutations, there exists a small fraction of beneficial mutations that play a decisive role in species evolution. These beneficial effects drive the mutation rate upwards. Another factor affecting the mutation rate is the expenditure of energy and time, referring to the cellular energy and time spent on replication proofreading and damage repair. If the mutation rate is lower, this expenditure becomes more significant, hence driving the mutation rate upwards. Presently, evolutionary models on mutation rates include classical models that consider all three driving forces and the drift-barrier model that focuses solely on harmful mutations. Studies in yeast have revealed that upon the removal of natural selection, the mutation rate may either increase or decrease. This finding aligns with classical models, negating the drift-barrier hypothesis.
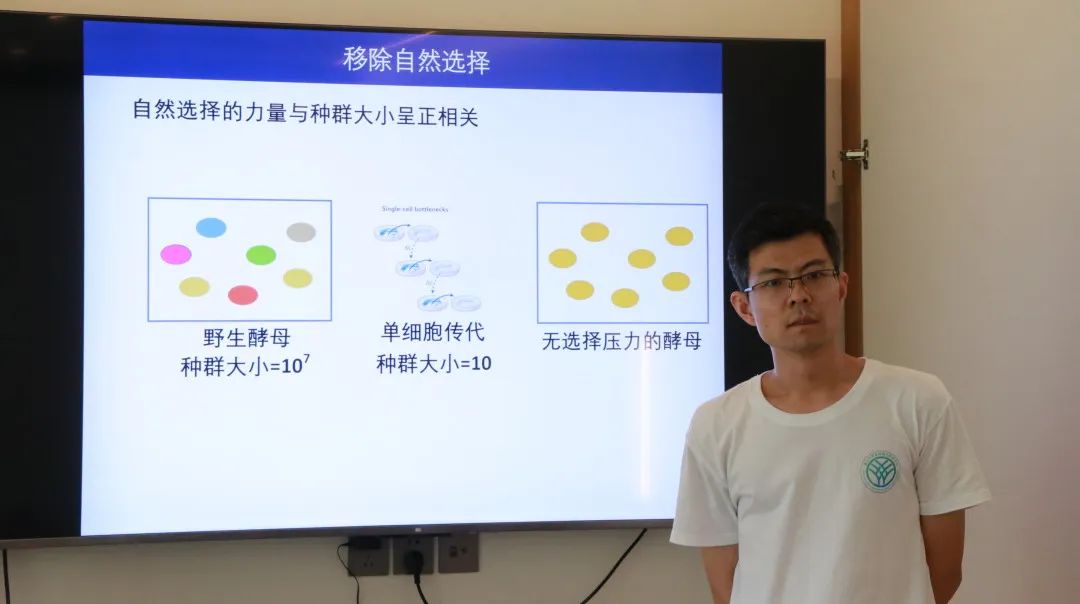
Dr. Haoxuan Liu from Center for Evolutionary & Organismal Biology
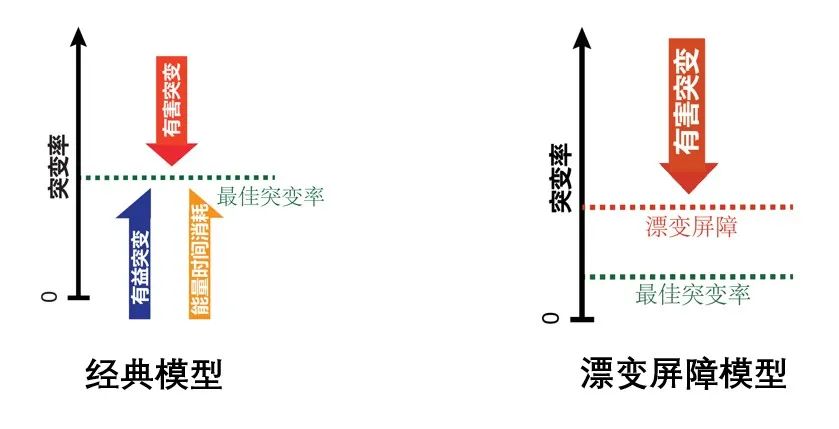
The classic models of mutation rate evolution and the drift barrier model
Broadly, mutations don't only refer to DNA variations but also encompass changes in the cellular epigenetic regulatory levels. Such variations also carry the potential for heredity. Dr. Ying Zhang from Beijing Normal University presented advancements in research on this type of acquired heredity. In experiments, mice exposed to a high-fat diet exhibited an obese phenotype. Surprisingly, the descendants of obese mice, even after being removed from the high-fat diet, also showed a tendency toward obesity. Unexpectedly, genomic sequencing of obese mice did not reveal mutations at the DNA level. Further research suggests this phenomenon might be due to acquired heredity mediated by tRNA within sperm cells.

Dr. Ying Zhang from Beijing Normal University
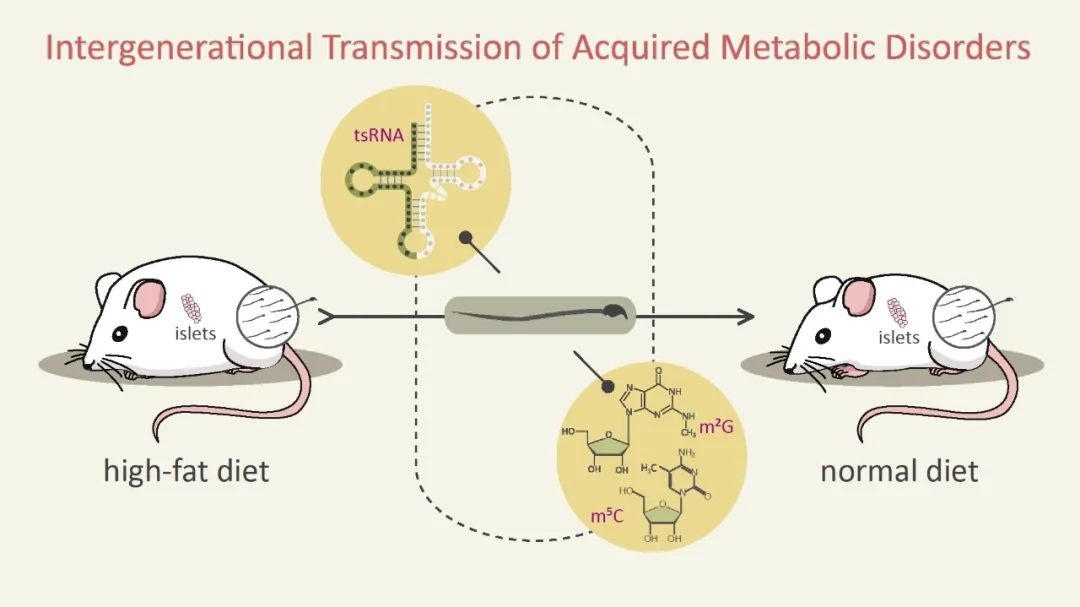
Paternal obesity inheritance in mice
Dr. Jing Qu from the Institute of Zoology, Chinese Academy of Sciences, presented the mechanisms of aging and regenerative interventions. She discussed recent research findings across three main areas: the aging platform of stem cells, aging biomarkers, and interventions for aging.

Dr. Jing Qu from the Institute of Zoology, Chinese Academy of Sciences
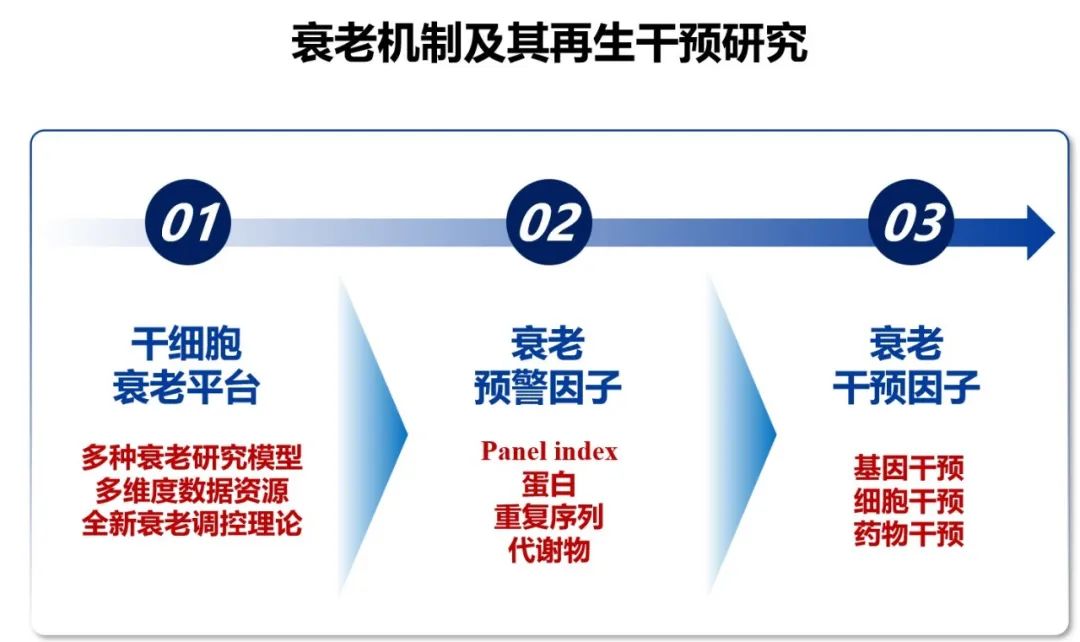
The research directions of aging mechanisms
Large-scale mutations, such as recombination errors, replication errors, or transposon activity, can lead to extensive DNA segment duplications. This mechanism also serves as a significant driving force for the emergence of new genes. Dr. Yong Zhang from the Institute of Zoology, Chinese Academy of Sciences, presented the latest research advancements in this area. Dr. Zhang's team uncovered gene duplication events driven by mutation biases through genomic analyses of humans and fruit flies. By mining extensive genomic data, they identified that gene duplications mediated by transposons are widely prevalent.

Dr. Yong Zhang from the Institute of Zoology, Chinese Academy of Sciences
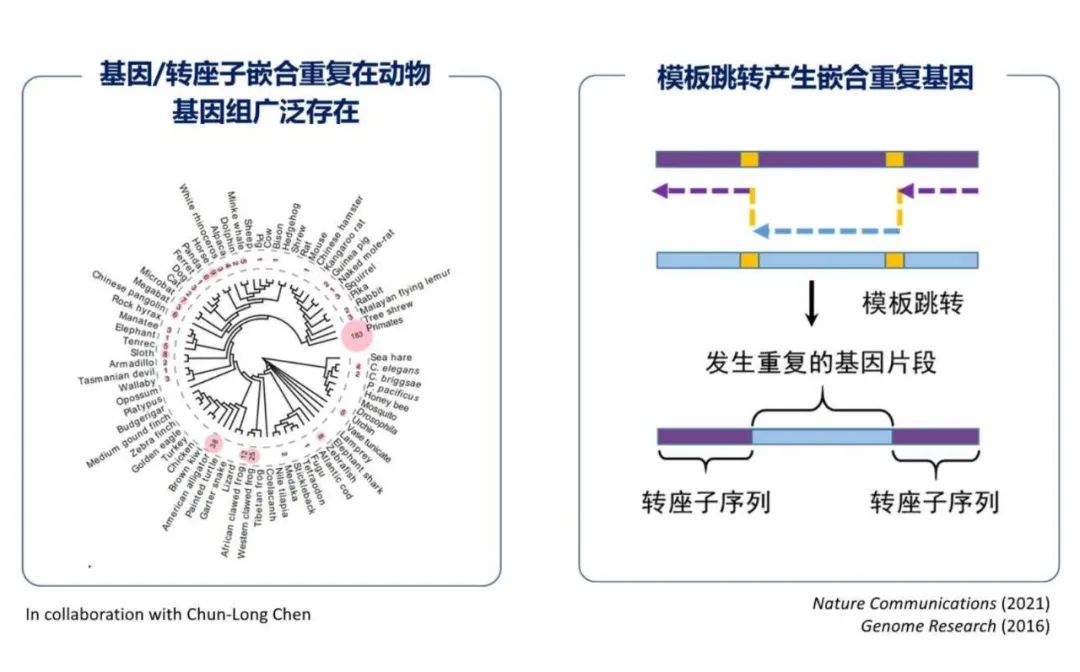
Transposon-mediated gene duplications
Apart from DNA, histones and their methylation can also be inherited. There are various modifications on histones that play a crucial role in regulating cell fate. Dr. Dong Fang from the Life Sciences Institute, Zhejiang University, highlighted recent advancements in this field, delving into the mechanisms behind oncogenic histone-induced cancer formation.

Dr. Dong Fang from the Life Sciences Institute, Zhejiang University
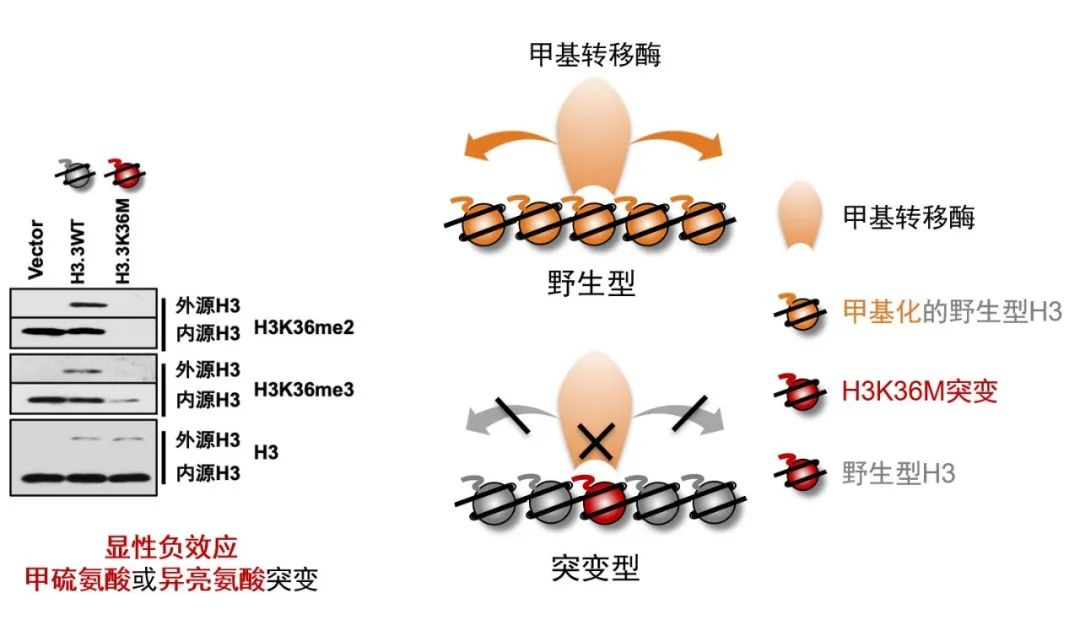
The mechanism proto-oncogenes induce the formation of cancer
Mutations are the fundamental source of species diversity. Dr. Shaohong Feng from the Center for Evolutionary& Organismal Biology at Zhejiang University presented the Avian Genomics Project, which gathered omics data, embryonic development data, phenotypic data, and geographical data. On this basis, they studied the genetic variation underlying beak morphology, brain volume, wing morphology, and egg shape, establishing models for climate response mediated by these traits.

Dr. Shaohong Feng from the Center for Evolutionary& Organismal Biology, Zhejiang University
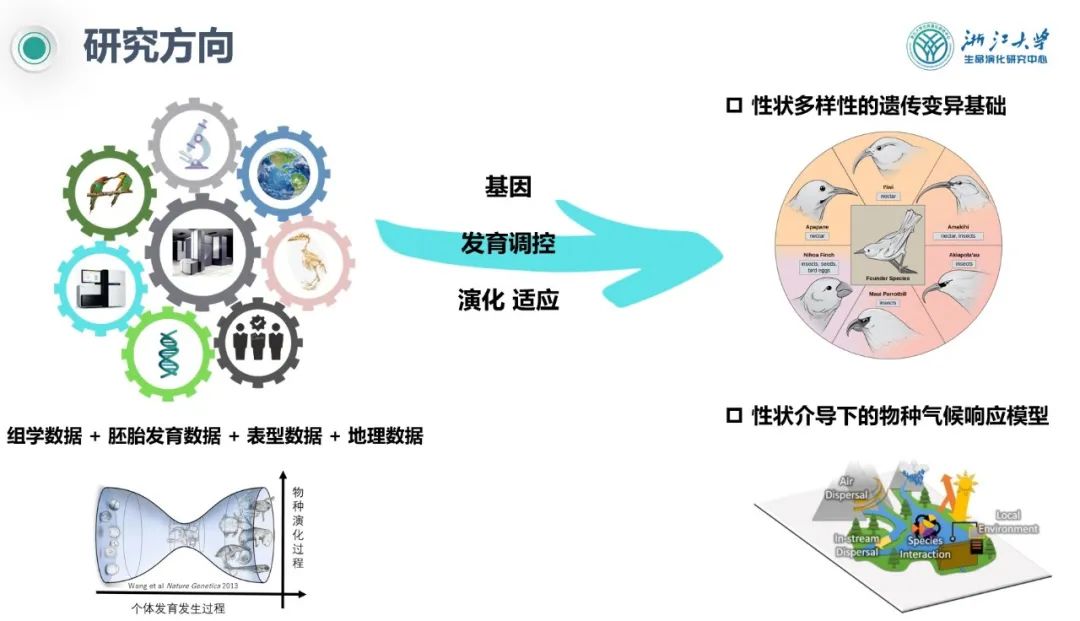
The large-scale collection of data and research directions within avian species
Discussion of Questions
After presenting their respective fields' latest research achievements, the teachers engaged in an intense discussion on the following inquiries:
1. Mechanisms explaining the significantly lower mutation count in germ cells compared to somatic cells.
In humans, approximately 70 mutations are transmitted to the offspring through germ cells, whereas other somatic cells carry around 1000 to 4000 mutations per cell. The reasons for this disparity remain unclear.
2. The cause behind the threefold higher reproductive mutation count in males compared to females.
Known as "male-driven evolution" in evolutionary biology, this phenomenon has two explanations. Firstly, sperm undergoes more divisions than eggs, leading to a higher accumulation of mutations. Secondly, due to physiological structure and gene expression differences, the male reproductive system is more prone to environmental stimuli-induced mutations. Despite male reproductive cells continually dividing as males age, the ratio of mutation rates between male and female reproductive cells consistently maintains a 3:1 ratio across different ages, contrary to expectations.
3. The relationship between somatic cell mutations and cancer.
Research indicates a positive correlation between somatic cell mutation rates and the probability of cancer occurrence among different species. However, it remains unclear whether a high mutation rate directly causes cancer.